Guyton Figure 40.8
Readings REQUIRED or RECOMMENDED: Guyton and Hall (9th edition). Chap. 40; Ganong (19th edition), Chap. 35
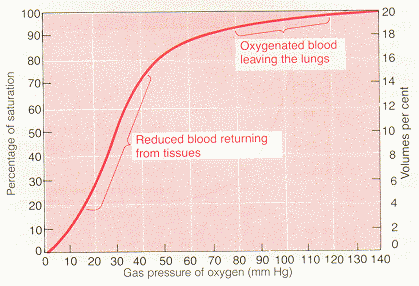
oxygen dissociation curve: The curve relating percentage saturation of the O2 carrying power of hemoglobin to the PO2, has a characteristic sigmoid shape.
oxyhemoglobin: Hemoglobin with O2 attached. The oxygen molecule combines loosely and reversibly with the heme portion of the hemoglobin (high affinity conformation).
deoxyhemoglobin: Hemoglobin after the oxygen is released (low affinity conformation).
oxygen saturation: The degree to which O2 binding sites are occupied or filled on the hemoglobin.
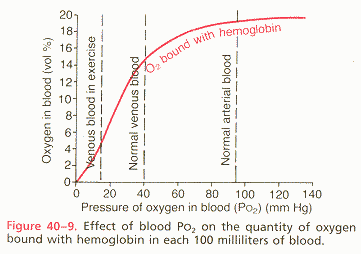
oxygen carrying capacity: The O2 carrying capacity of Hb=1.34 ml O2/gm Hb. Therefore a person with the "normal" 15 gm Hb per 100ml blood has a Hb O2 carrying capacity of 20.1 ml O2/100ml blood. (100% O2 saturation at 250 mmHg PO2)
cooperativity: A change in oxygen affinity to hemoglobin, making it easier for more O2 to "hop on". When none of the iron-binding sites is occupied, Hb is in a T (tense) state and not receptive to O2. But once an O2 does bind to one site, the iron moves slightly and so do parts of the polypeptide chain attached to it, making it progressively easier for the next O2, until all four sites are occupied by O2 and the Hb is in an R ("relaxed") state. This cooperative behavior facilitates O2 loading in the lungs. Conversely (in the tissues) as one O2 frees itself from the Hb, the Hb changes, making it easier for the next to unload.
oxygen affinity: The ease with which oxygen binds with hemoglobin or is released from the hemoglobin.
P50: An index of shifts in the oxy-hemoglobin dissociation curve, the PO2 at which hemoglobin is half saturated with O2. The higher the P50, the lower the affinity of hemoglobin for O2.
hematocrit: Fraction of the blood composed of red blood cells, as determined by centrifuging blood in a "hematocrit tube" until the cells become tightly packed in the bottom of the tube, makes up about 45% of the blood volume.
2,3 diphosphoglycerate: A phosphorus-containing metabolite found in red cells. It is a highly charged anion that binds to the B chains of deoxyhemoglobin. It stabilizes hemoglobin in a low affinity state, facilitating O2 delivery (an increase in the concentration of 2,3 DPG shifts the reaction to the right). Red cell 2,3 DPG concentration is increased with ascent to high altitude, as well as in anemia and a variety of diseases in which there is a chronic hypoxia; this facilitates the delivery of O2 to the tissues--P50 is stabilized.
myoglobin: Iron-containing pigment--it resembles hemoglobin but binds one rather than four molecules of O2. Its curve is to the left of the Hb curve--takes up O2 from Hb in the blood. It releases O2 only at low PO2 values.
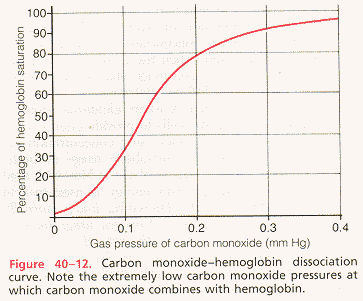
carbon monoxide: Chemical compound of carbon and oxygen, with the formula CO. It has approximately 200 times greater affinity for Hb than oxygen, it will always bind before oxygen can. Normal levels of HbCO (carboxyhemoglobin) are less then 2% and in cigarette smokers may be up to 10%. The body cannot use carbon monoxide for metabolism; so when enough Hb becomes bound with carbon monoxide and not oxygen, tissue hypoxia will result.
carboxyhemoglobin: Formed when carbon monoxide reacts with hemoglobin. The affinity of Hb for O2 is much lower than its affinity for CO, which consequently displaces O2 on Hb, reducing the oxygen carrying capacity of blood.
carbon monoxide poisoning: Symptoms of anemic hypoxia appear in carbon monoxide poisoning because carbon monoxide competes with O2 for binding sites on the Hb molecule, reducing the amount of Hb available to carry O2 (Hb's affinity for carbon monoxide is about 200 times greater than its affinity for O2).
arterio-venous oxygen content difference: Under normal conditions, about 5 milliliters of oxygen are transported to the tissues by each 100ml of blood (5ml O2/dl).
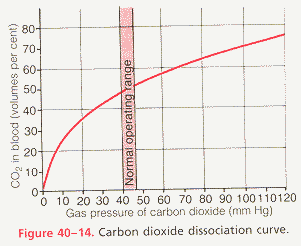
dissolved C02: The amount of carbon dioxide dissolved in the fluid of the blood at 45 mmHg is about 2.7 ml/dl. The amount dissolved at 40 mmHg is about 2.4 ml, or a difference of .3 milliliter. Therefore, only about .3ml of carbon dioxide is transported in the form of dissolved carbon dioxide by each deciliter of blood (about 7% of all the carbon dioxide transported).
bicarbonate ion: The carbonic acid formed in the red cells dissociates into bicarbonate (and hydrogen) ions. Many of the bicarbonate ions diffuse from the red cells into the plasma while chloride ions diffuse into the red cells to take their place (made possible by bicarbonate-chloride carrier protein in the red cell membrane that shuttles these ions in opposite directions).
carbaminohemoglobin: (CO2Hgb) Compound which is formed when carbon dioxide reacts directly with amine radicals of the hemoglobin molecules. This combination of carbon dioxide with the hemoglobin is a reversible reaction that occurs with a loose bond, so that the CO2 is easily released into the alveoli where the PCO2 is lower than in the tissue capillaries.
carbonic anhydrase: An enzyme which catalyzes the reaction between carbon dioxide and water, accelerating its rate by approx. 5,000 times. This allows tremendous amounts of carbon dioxide to react with the red cell water even before the blood leaves the tissue capillaries.
chloride shift: The chloride content of venous red blood cells is greater than that of arterial red cells.
CO2 dissociation curve: Curve which graphically depicts the dependence of total blood carbon dioxide in all its forms on PCO2.
arterio-venous carbon dioxide content difference: Under normal resting conditions, an average of 4 milliliters of carbon dioxide is transported from the tissues to the lungs in each deciliter of blood.
describe the two modes of transport of oxygen by blood and discuss their relative magnitudes and significance: Normally, approx. 97% of the oxygen transported from the lungs to the tissues is carried in chemical combination with hemoglobin in the red blood cells. The remaining 3 per cent is carried in the dissolved state in the water of the plasma and cells. Therefore, under normal conditions, oxygen is carried to the tissues almost entirely by hemoglobin. The oxygen molecule combines loosely and reversibly with the heme portion of the hemoglobin. When the PO2 is high, as in the pulmonary capillaries, oxygen binds with the hemoglobin, but when the PO2 is low, as in the tissue capillaries, oxygen is released from the hemoglobin. This is the basis for almost all oxygen transport from the lungs to the tissues. At the normal arterial PO2 of 95 mmHg, about .29 ml of oxygen is dissolved in every deciliter of water in the blood. Then when the PO2 of the blood falls to 40 mmHg in the tissue capillaries, only .12 ml of oxygen remains dissolved. In other words, .17 ml of oxygen is normally transported in the dissolved state to the tissues by each deciliter of blood. This compares with almost 5 ml transported by the hemoglobin. Therefore, transport of oxygen in the dissolved state is considerably less significant physiologically.
draw a reasonably accurate representation of the normal oxygen-hemoglobin equilibrium curve with appropriate axes and accurate values, and locate on this curve typical arterial and venous points, and indicate the arteriovenous oxygen content difference: See figures below

Guyton Figure 40.8
Guyton
discuss the origin of the sigmoidal shape of the oxyhemoglobin dissociation curve, and state its significance for oxygen uptake and delivery: The curve is S-shaped because the hemoglobin has four subunits, therefore each Hb can carry four O2 molecules. Most of its O2 is unloaded in a very narrow range of PO2 (between 20 and 40 mmHg). This behavior is due to the cooperative nature of O2 binding to Hb. The first portion of the curve at very low PO2 is flat because Hb is in the "tense" state and not receptive to O2. As more O2 are introduced, the likelihood of one of them binding goes up. Once it binds it further increases the probabiltiy of successive oxygen binding. Therefore, the binding (saturation) curve rises very steeply and in just the right region. This S-shape has great physiological significance.
differentiate clearly among the following terms and be able to define and state the units for each: oxygen partial pressure, oxygen saturation, oxygen content, oxygen carrying capacity:
identify four factors that affect hemoglobin-oxygen affinity and shift the position of the oxygen dissociation curve, indicate whether each of the factors is involved in acute or chronic shifts in oxygen affinity, and discuss the role of each in gas exchange in the lung or tissues:
describe in words or on a graph the effects of carbon monoxide poisoning on the shape and position of the oxygen-hemoglobin equilibrium curve and on tissue oxygen delivery: See figure below.
Guyton
name and describe the three modes of transport of carbon dioxide by blood and their relative magnitudes and significance:
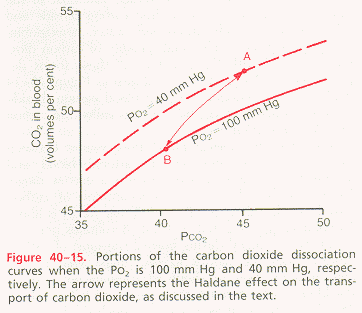
draw the carbon dioxide dissociation curve with appropriate axes and values, locate typical arterial and venous points, and discuss the significance of this curve for carbon dioxide transport: See figures below.
Guyton
Guyton
discuss the compensatory changes in the oxygen transport system that occur in the presence of arterial hypoxemia, regardless of its cause:
Last Updated 04/10/00 12:27:11 PM
Return To The MNA 2001 Homepage