 Figure 1 ( i.)
Figure 1 ( i.)Adiabatic Expansion of a Gas
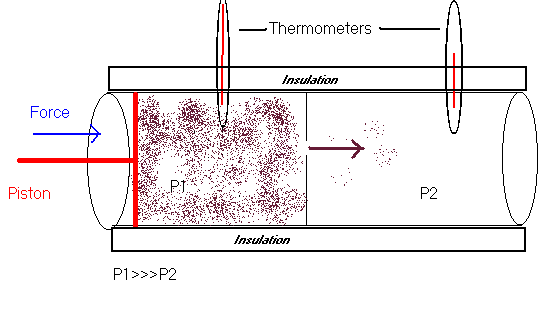
Adiabatic expansion of a gas can be described by imagining a cylinder of gas (see figure) that is closed off from the environment and is insulated so that it can NOT be heated or cooled by any external sources (e.g. fire, ice, water, electricity). On the left side of the cylinder there is a gas under pressure (P1); the pressure is created by pushing on a piston. On the right side there is an area of very low pressure or a vacuum. The two sides of the cylinder are separated by a partition that has a small hole (throttle) in it. As the gas passes through the throttle from the high-pressure side (left side) to the low-pressure side (right side) it expands very rapidly. This is adiabatic expansion.
 Figure 1 ( i.)
Figure 1 ( i.)
Everything has an internal energy, even gases. When pressure is applied to a gas its internal energy increases because we are performing work on the gas. As the gas expands its internal energy decreases because the gas is performing work by expanding.
(1) D internal energy (D U) = heat added or removed (dq) + work done to or by the gas.
Since there is no heat added or removed, the first term (dq) = 0. So (1) becomes
(2) D internal energy (D U) = work done by the gas (dw)(ii)
When gas performs work dw is negative so the internal gas decreases. Intuitively since we know that temperature is an indirect measurement of energy, we can guess that the temperature of the gas will decrease as it expands. This decrease in temperature is the Joules -- Thompson effect.
This is how your refidgerator or AC works. A compressor (left side of cylinder) pumps a gas (freon) through a throttle and into a network of coils (right side of cylinder) where it rapidly expands and cools.
Return to the MNA 2001 homepage
Last updated 04/10/00 12:26:45 PM